HARD
Earn 100
Identify in which of the following work is done on system or by the system.
Important Questions on Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
(R = 8.314 J/mol K) (ln7.5 = 2.01)
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
The specific heat of a certain substance is . Assuming ideal solution behavior, the energy required (in ) to heat of molal of its aqueous solution from to is closest to :
[Given: molar mass of the substance ; specific heat of water ]
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
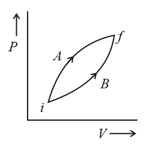
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other

