Identify the correct statements when a little amount of is added to solution

Important Questions on Solutions
A closed jar having water vapours in equilibrium with liquid suddenly all the vapours of jar is transferred to another identical jar and is subjected to compression at same temperature and negligible volume occupied by the liquid water. Select the observation in the second jar.
A beaker containing mole of in of and a beaker containing mole of in of are placed in a chamber and allowed to equilibrate. What is the concentration (mole fraction) of in the resulting solutions?
moles of gas dissolves completely in water at , as shown in the following figure.
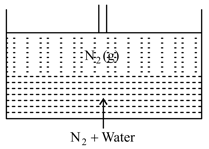
The available space between piston and liquid solution is . Find the number of moles of in gaseous phase.
for gas , Round off the value to the nearest integer.
In an Ostwald-Walker experiment, dry air was first blown through a solution containing a certain amount of solute in of water and then also through pure water. The loss in mass of water was found to be , while the mass of water absorbed in sulphuric acid was . Calculate the amount of the solute.
Give your answer up to two decimal places without rounding-off.
To of water, of acetic acid is added. If of acetic acid is dissociated, what will be the depression in freezing point? and density of water are and respectively.
Give answer after multiplying with 100 and rounding off to the nearest integer value.
