EASY
Earn 100
Identify the factor from the following that does not affect electrolytic conductance of a solution.
(a)The nature of the electrolyte added.
(b)The nature of the electrode used.
(c)Concentration of the electrolyte.
(d)The nature of solvent used.
58.97% studentsanswered this correctly
Important Questions on Redox Reactions and Electrochemistry
MEDIUM
What is the molar conductivity of if its conductivity is ?
MEDIUM
Which of the following electrolytic solution has the least specific conductance
EASY
The pair of electrolytes that possess same value for the constant in the Debye - Huckel - Onsagar equation, is
EASY
What are the units of equivalent conductivity of a solution?
HARD
Resistance of 0.2 M solution of an electrolyte is . The specific conductance of the solution is . The resistance of 0.5 M solution of the same electrolyte is . The molar conductivity of 0.5 M solution of the electrolyte in is :
MEDIUM
At a particular temperature, the ratio of molar conductance to specific conductance of solution is
EASY
The variation of molar conductively with concentration of an electrolyte (X) in aqueous solution is shown in the given figure.
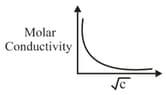
The electrolyte X is :
MEDIUM
Given below are two statements:
Statement I : The limiting molar conductivity of (strong electrolyte) is higher compared to that of $\mathrm{CH}_{3} \mathrm{COOH}$ (weak electrolyte).
Statement II : Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
EASY
Aqueous solution of which of the following compounds is the best conductor of electric current?
HARD
Which one of the following graphs between molar conductivity versus is correct?
EASY
The molar conductivities of and at infinite dilution are and respectively. The molar conductivity of . at infinite dilution will be
HARD
The equivalent conductance of at concentration C and at infinite dilution are and , respectively. The correct relationship between and is given as :
(where the constant B is positive)
EASY
If the resistivity of solution is What is its molar conductivity?
HARD
Among the following, the plot that correctly represents the conductometric titration of with is
EASY
The resistance of solution at is If the conductivity of solution at is The cell constant of the conductivity cell in is
MEDIUM
The resistance of a conductivity cell containing solution at is . What is the cell constant if the conductivity of solution at is
MEDIUM
Match List - I with List - II :
| List - I (Parameter) |
List - II (Unit) | ||
| (a) | Cell constant | (i) | |
| (b) | Molar conductivity | (ii) | Dimensionless |
| (c) | Conductivity | (iii) | |
| (d) | Degree of dissociation of electrolyte | (iv) |
EASY
Consider the statements and :
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
MEDIUM
The molar conductivities of and at infinite dilution follow the order
MEDIUM
The molar conductivity of a solution of with electrolytic conductivity of at

