MEDIUM
Earn 100
Identify the incorrect statements for an ideal gas.
(a)The product of the volume and the pressure of a fixed amount of gas is dependent on the temperature.
(b)Molecules of different gases have the same 'Average Kinetic Energy' at a given
temperature.
temperature.
(c)Van der Waals interaction dominates over thermal energy at a very low temperature.
(d)Collision of molecules is perfectly elastic.
50% studentsanswered this correctly
Important Questions on States of Matter
EASY
MEDIUM
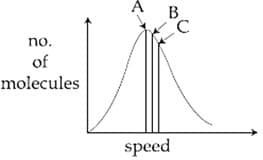
Root mean square speed most proable speed Average speed
EASY
MEDIUM
EASY
MEDIUM
HARD
If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
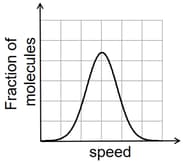
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
EASY

