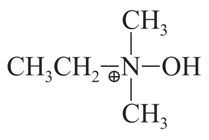
Identify the major product in the following reaction?
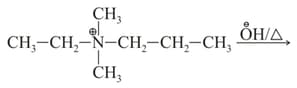
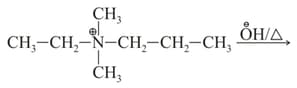

Important Questions on Amines
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Amides react with in presence of to form -amine having one carbon atom less than amide.
It is degradative reduction involving -bromoalkanamide intermediate.
Directions: Each of these questions contain an assertion followed by reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Assertion: Nitrobenzene and aniline do not undergo Friedel-Craft's reaction with .
Reason: Moderate activated ring always shows Friedel-Craft's reaction.
Directions: Each of these questions contain an assertion followed by reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Assertion: Nitration of aniline is generally carried out after protecting amino group by acetylation.
Reason: Acetylation decreases the activating effect of amino group.
Directions: Each of these questions contain an assertion followed by reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Assertion: Benzene diazonium chloride undergoes coupling reaction with , -dimethyl aniline to form an azodye, but it does not couple with - dimethyl-, -dimethyl aniline.
Reason: A para position of -dimethyl-, -dimethyl aniline is not sufficiently activated for coupling reaction.
Directions: Each of these questions contain an assertion followed by reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Assertion: In a strongly acidic solution, aniline becomes less reactive towards electrophilic reagents.
Reason: The amino group is completely protonated in a strongly acidic solution and the lone pair of electrons on the nitrogen are no longer available for resonance.
Assertion: Benzenediazonium ion couples with aniline effectively in a weakly acidic medium.
Reason: In a weakly acidic medium, aniline becomes respectively more activated for electrophilic substitution.
Assertion: Aniline reacts with concentrated to form anilinium sulphate, which on heating to about forms mainly -aminobenzene suiphonic acid (suiphanilic acid).
Reason: Sulphanilic acid exists as a dipolar ion.
Directions: Each of these questions contain an assertion followed by reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Assertion: In order to convert to pure , Gabriel phthalimide synthesis can be used.
Reason: With proper choice of alkyl halides, phthalimide synthesis can be used to prepare or amines.
