HARD
JEE Main/Advance
IMPORTANT
Earn 100
Identify true and false statements for fixed amount of gas in following isotherm of real gas.
(i) From point to point volume is constant and temperature is increasing.
(ii) From point to point pressure is constant and temperature is decreasing.
(iii) Through path we can convert gas into liquid through continuity of state.
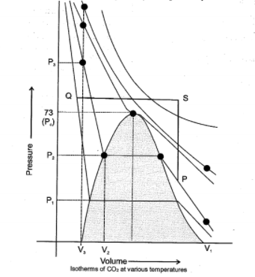
(i) From point to point volume is constant and temperature is increasing.
(ii) From point to point pressure is constant and temperature is decreasing.
(iii) Through path we can convert gas into liquid through continuity of state.


Important Questions on States of Matter: Gases and Liquids
MEDIUM
JEE Main/Advance
IMPORTANT
Explain the physical significance of Van der Waal's parameters.
MEDIUM
JEE Main/Advance
IMPORTANT
The critical temperature and critical pressure of a gas are and atmospheres respectively.
Calculate the constants and
MEDIUM
JEE Main/Advance
IMPORTANT
Calculate the volume occupied by mole of at and atm pressure,
if and .
HARD
JEE Main/Advance
IMPORTANT
Using the Van der Waals equation, calculate the pressure of gas in a vessel at .
MEDIUM
JEE Main/Advance
IMPORTANT
If density of vapours of a substance of molar mass at atm pressure and is , calculate the value of for the vapours. (Take )
MEDIUM
JEE Main/Advance
IMPORTANT
One litre gas at and atm pressure is compressed to a pressure of atm and . The compressibility factor is changed from to , respectively. Calculate the final volume of the gas.
MEDIUM
JEE Main/Advance
IMPORTANT
Reduced temperature for benzene is and its reduced volume is Calculate the reduced pressure of benzene.
EASY
JEE Main/Advance
IMPORTANT
Consider a real gas placed in a container. If the intermolecular attractions are supposed to disappear suddenly, which of the following would happen?
