MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
If amount of heat energy is provided to a monoatomic gas sample having two moles in a process constant, then increase in temperature of gas is equal to
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
A monoatomic gas (one mole) is taken through process as shown in figure. Find average molar heat capacity for the process.
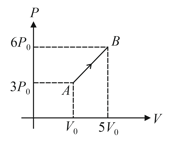
EASY
JEE Main/Advance
IMPORTANT
Consider the diagram shown in figure, value of work done by gas is given by
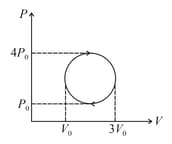
HARD
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
Find net work done by gas in the cyclic process
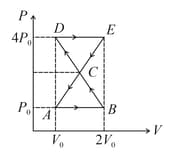
EASY
JEE Main/Advance
IMPORTANT
An ideal monatomic gas undergoes process as shown. Find efficiency of the thermodynamic cyclic shown in figure.
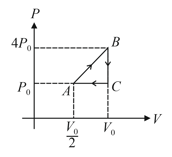
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
