EASY
Earn 100
If is the intensity of absorbed light and is the concentration of for the photochemical process , the rate of formation of is directly proportional to
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
HARD
(Assume that all these gases behave as ideal gases)
EASY
HARD
For an elementary chemical reaction, , the expression for is:
EASY
Rate
If the concentration of A is kept the same but that of B is doubled what will happen to the rate itself?
MEDIUM
HARD
MEDIUM
Which one of the following statements is correct?
EASY
HARD
(i) time, (ii) concentration of reactants, (iii) temperature, and (iv) catalyst
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
(i)
(ii)
(iii)
The overall order of the reaction will be
HARD
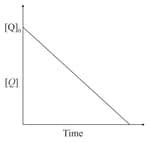
the time taken for reaction of is twice the time taken for reaction of . The concentration of varies with reaction time as shown in the figure.The overall order of the reaction is:
MEDIUM
EASY
EASY

