EASY
Earn 100
If 10-4 dm3 of water is introduced into a 1.0 dm3 flask at 300 K, how many moles of water are in the vapour phases when equilibrium is established?
(Given : Vapour pressure of H2O at 300 K is 3170 Pa ; R = 8.314 J K-1 mol-1 )
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on States of Matter
HARD
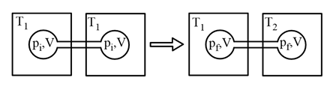
EASY
MEDIUM
MEDIUM
EASY
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
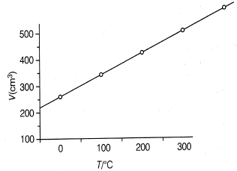
The volume of the gas at is larger than that at by a factor of
EASY
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
HARD
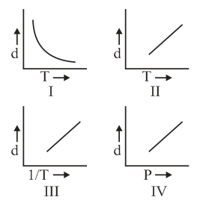
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
MEDIUM
HARD
MEDIUM
[Gas constant, ]
MEDIUM
MEDIUM
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?

