EASY
JEE Main/Advance
IMPORTANT
Earn 100
If mole, mole of two ideal gases having adiabatic constant and respectively are mixed, then for the mixture will be
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
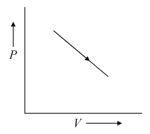
If the indicator diagram for expansion of gas is as shown, the gas
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
