MEDIUM
JEE Main
IMPORTANT
Earn 100
If molecule is a polar molecule, a possible geometry of is :
(a)Square pyramidal
(b)Tetrahedral
(c)Rectangular planar
(d)Square planar
40% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Main
IMPORTANT
Which one of the following molecules is paramagnetic?
MEDIUM
JEE Main
IMPORTANT
Amongst LiCl, RbCl, BeCl2 and MgCl2 the compounds with the greatest and the least ionic character, respectively are :
EASY
JEE Main
IMPORTANT
Which of the following conversions involves change in both shape and hybridisation?
MEDIUM
JEE Main
IMPORTANT
The incorrect geometry is represented by:
EASY
JEE Main
IMPORTANT
In the molecular orbital diagram for the molecular ion , the number of electrons in the molecular orbital is
MEDIUM
JEE Main
IMPORTANT
Identify the pair in which the shape of the species is T-shaped and square-pyramidal, respectively,
MEDIUM
JEE Main
IMPORTANT
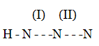
The above compound represents hydrogen azide, the bond orders of bonds and are:
MEDIUM
JEE Main
IMPORTANT
In graphite and diamond, the percentage of p-characters of the hybrid orbitals in hybridization respectively, are
