If an atom contains one electron and one proton, what charge will it carry?
Important Questions on Structure of the Atom
Who led to the discovery of canal rays?
Identify the difference between particles of each given pair.
(I) and
(II) and
(III) and
| I | II | III | |
| (A) | Number of protons | Number of electrons | Number of neutrons |
| (B) | Number of electrons | Number of protons | Number of neutrons |
| (C) | Number of neutrons | Number of electrons | Number of protons |
| (D) | Number of protons | Number of neutrons | Number of electrons |
Study the given table carefully and select the incorrect statement(s).
| Element | Number of protons | Number of neutrons | Number of electrons |
I. and are cations.
II. is the lightest element while is the heaviest element.
Ill. is a noble gas.
IV. and are anions.
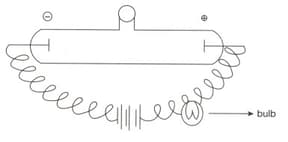
A certain amount of gas is enclosed in a discharge tube. The bulb in the arrangement given below can be made to glow when:
Electrons and protons are represented as?
Three invisible radiations and are passed through an electric field. goes straight, deviates towards the positive end while deviates towards the negative end. The particles present in and and the ratio for are
| ratio for | ||||
| (A) | -Particles | -Particles | -Rays | |
| (B) | Neutrons | Electrons | Protons | |
| (C) | -Particles | -Particles | -Rays | |
| (D) | Neutrons | Electrons | Protons |
Description of a few atoms is given in the table.
| Atom | ||||
| No. of protons | ||||
| No. of neutrons | ||||
| No. of electrons |
Identify a cation, an anion and a pair of isotopes from the given table:
| Cation | Anion | Pair of isotopes | |
| (A) | |||
| (B) | |||
| (C) | |||
| (D) |
Which of the following is true for mass and charge of electron?
Which of the following is true for mass and charge of proton?
Which of the given pairs of atoms contain(s) the same number of neutrons?
I and
II and
III and
IV and

