EASY
Earn 100
If an element in group formed a compound with an element in group of the periodic table, the compound formed is likely to _______
(a)conduct electricity in the solid state
(b)have a low boiling point
(c)dissolve in non-polar solvents
(d)be a crystalline solid
75% studentsanswered this correctly
Important Questions on Solid State
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is
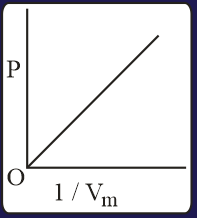
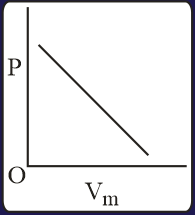
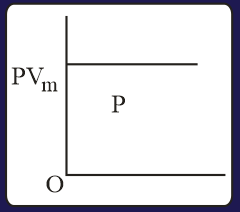
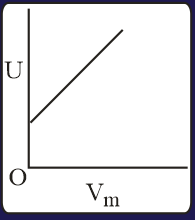
HARD
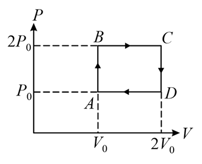
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
EASY
EASY
EASY
HARD
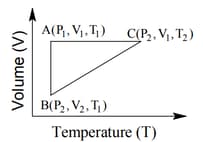
The correct option(s) is (are)
MEDIUM
EASY
(Latent heat of ice is and )
MEDIUM
MEDIUM
[Heat of fusion of ice ; Specific heat of water ]
MEDIUM
(A) Crystalline solids have long range order.
(B) Crystalline solids are isotropic.
(C) Amorphous solid are sometimes called pseudo solids.
(D) Amorphous solids soften over a range of
temperatures.
(E) Amorphous solids have a definite heat of fusion. Choose the most appropriate answer from the options given below.
EASY
MEDIUM
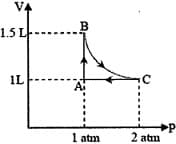
EASY
HARD
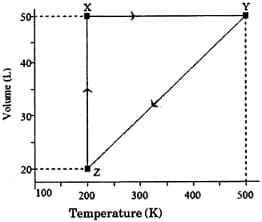
The pressure of the gas (in atm) at and respectively, are
MEDIUM
HARD
MEDIUM
Given below are two statements. One is labelled as
Assertion and the other is labelled as Reason .
Assertion Sharp glass edge becomes smooth on heating it up to its melting point.
Reason The viscosity of glass decreases on melting.
Choose the most appropriate answer from the options given below.
EASY

