EASY
NEET
IMPORTANT
Earn 100
If an ideal flask containing hot coffee is shaken, the temperature of the coffee will:
(a)decrease
(b)increase
(c)remain same
(d)decrease if temperature is below and increase if temperature is equal to or more than .
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
An ideal gas undergoes a cyclic process as shown in the given diagram, where the process is adiabatic. The process is also represented by
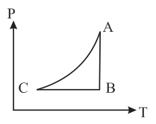
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
Two samples of air and having same composition and initially at same temperature , pressure and volume are taken. and are made to undergo the following process:
Case and are compressed from volume to volume is compressed isothermally while is compressed adiabatically. The final pressure are and , respectively.
Case: and are allowed to undergo expansion from volume to volume . undergoes isothermal expansion while undergoes adiabatic expansion. The final pressures of and are and , respectively.
