EASY
NEET
IMPORTANT
Earn 100
If degree of dissociation of is in a solvent, then
(a)Normal boiling point = experimental boiling point
(b)Normal osmotic pressure experimental osmotic pressure
(c)Normal molecular weight experimental molecular weight
(d)Normal freezing point experimental freezing point
66.67% studentsanswered this correctly

Important Questions on Solutions
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
I. Molarity
II. Molality
III. Normality
IV. Mole fraction
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Vapour phase diagram for a solution is given below if dotted line represents deviation
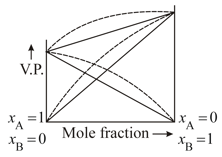
Correct observation for this solution
EASY
NEET
IMPORTANT
A mixture of two liquidsand having boiling point of is , and boiling point of is , distills at as single liquid, hence this mixture is
