MEDIUM
JEE Main
IMPORTANT
Earn 100
If ideal diatomic gas follows the process as shown in graph where is temperature in and is volume , then molar heat capacity for this process will be [in terms of gas constant ],
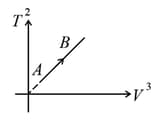

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main
IMPORTANT
Air in a cylinder is suddenly compressed by a piston, which is then maintained at the same position. With the passage of time,
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
Ideal gas is taken through a process as shown in figure
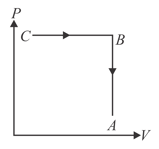
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
A closed vessel contains mole of a monoatomic ideal gas at If mole of the same gas at is added to it, the final equilibrium temperature (in ) of the gas in the vessel will be close to _______.
(Note: Round off the answer to nearest integer)
MEDIUM
JEE Main
IMPORTANT
