If one increases the volume of a soap bubble the surface tension of the bubble -
Important Questions on Mechanical Properties of Fluids
Calculate the work done in increasing the radius of a soap bubble in air from 1 cm to 2 cm. The surface tension of the soap solution is 30 dyne/cm.
(Surface tension of water
When water is filled carefully in a glass, one can fill it to a height above the rim of the glass due to the surface tension of water. To calculate just before water starts flowing, model the shape of the water above the rim as a disc of thickness having semicircular edges, as shown schematically in the figure. When the pressure of water at the bottom of this disc exceeds what can be withstood due to the surface tension, the water surface breaks near the rim and water starts flowing from there. If the density of water, its surface tension and the acceleration due to gravity are and respectively, the value of (in ) is ________.
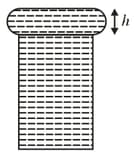
diameter of capillary,
rise of water,
Using and the simplified relation the possible error in surface tension is closest to:

