EASY
NEET
IMPORTANT
Earn 100
If one mole of the polyatomic gas is having two vibrational modes and is the ratio of molar specific heats for polyatomic gas then the value of is :
(a)
(b)
(c)
(d)
44% studentsanswered this correctly

Important Questions on Behaviour of Perfect Gas and Kinetic Theory
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Figure shows graphs of pressure vs density for an ideal gas at two temperatures and
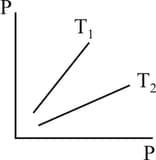
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
In the figure, the volume of container is double of the volume of container . Both are filled with ideal gas. The temperature of is and that of is . If the mass of gas in is , then the mass of gas in will be?

MEDIUM
NEET
IMPORTANT
