EASY
JEE Main/Advance
IMPORTANT
Earn 100
If pressure and temperature of an ideal gas are doubled and volume is halved, the number of molecules of gas
(a)Become half
(b)Become two times
(c)Become four times
(d)Remain constant
12.5% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
Pressure-temperature graph of the ideal gas at constant volume is shown by a straight line Now, pressure of the gas is doubled and the volume is halved; then the corresponding pressure temperature graph will be shown by the line
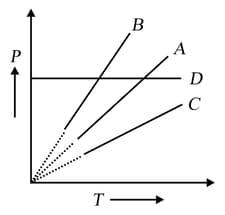
EASY
JEE Main/Advance
IMPORTANT
Two balloons are filled; one with pure gas and the other with air, respectively. If the pressure and temperature of these balloons are the same, then the number of molecules per unit volume is
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
The density versus pressure graphs of a given mass of an ideal gas is shown at two temperatures and Then relation between and may be
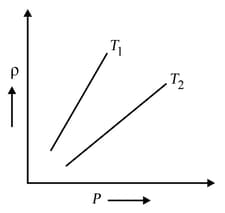
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
