If production technology improves, how is the market supply curve affected in a perfectly competitive market?
Important Questions on Straight Lines
For the given reaction, if the initial pressure is and the pressure at time is at a constant temperature and constant volume . The fraction of decomposed under these conditions is . The value of is (nearest integer)
When mole of and mole of are mixed in a closed container at a constant temperature, mole of is obtained at equilibrium. Calculate the equilibrium amount of and .
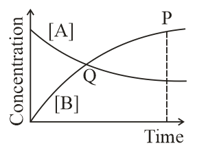
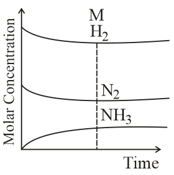
In the reaction , which of the graphs is/are correct?
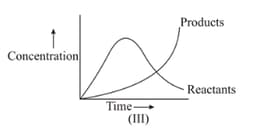
Among the following points indicated on the graph, which one represents equilibrium?
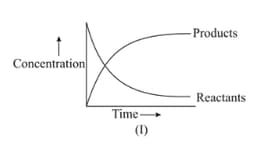
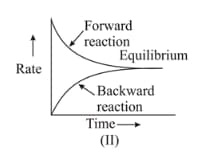
For the reaction , variation of concentration is plotted against time. The time at which the equilibrium establishes is as shown:
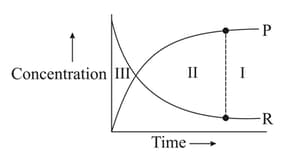
Which of the following regions show(s) equilibrium?
At equilibrium mixture for the reaction
2H2S ⇌2H2 + S2
had 1 mole of H2S, 0.2 mole of H2, and 0.8 mole of S2 in a
2 litre flask. The value of Kc in mol L- is
In terms of rate constants for forward and backward reactions (), equilibrium constant of a reaction is equal to _____.
(A)
(B)
(C)
Enter the correct answer as A, B or C.