MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
If rate constant is numerically the same for the three reactions of first, second and third order respectively. Assume all the reactions of the kind products. Which of the following is correct:
(a)if then
(b)if then
(c)if then
(d)All
50% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
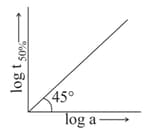
What will be the order of reaction and rate constant for a chemical change having . log concentration curves as ?
MEDIUM
JEE Main/Advance
IMPORTANT
For a reaction, product, rate law is
At a time when , concentration of the reactant is (initial concentration)
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
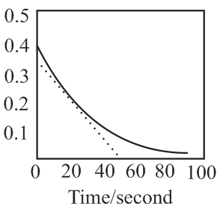
A reaction follows the given concentration-time graph. The rate for this reaction at seconds will be:
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
