EASY
NEET
IMPORTANT
Earn 100
If the concentration of reactants is increased by then rate constant becomes
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
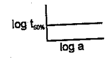
A graph plotted between . concentration is a straight line. What conclusion can you draw from this graph?
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
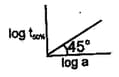
What will be the order of reaction and rate constant for a chemical change having a concentration of (A) curves as:
EASY
NEET
IMPORTANT
HARD
NEET
IMPORTANT
Which integrated equation is correct for the following order reaction started with only in a closed rigid vesset.
initial pressure; total pressure at time
