MEDIUM
JEE Main
IMPORTANT
Earn 100
If wavelength of the first line of the Paschen series of hydrogen atom is , then the wavelength of the second line of this series is _______ . (Nearest integer)
100% studentsanswered this correctly

Important Questions on Structure of Atom
MEDIUM
JEE Main
IMPORTANT
The number of s-electrons present in an ion with protons in its unipositive state is
EASY
JEE Main
IMPORTANT
Following figure shows spectrum of an ideal black body at four different temperatures. The number of correct statement/s from the following is _______.
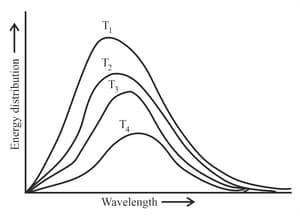
A. T4 > T3 > T2 > T1
B. The black body consists of particles performing simple harmonic motion.
C. The peak of the spectrum shifts to shorter wavelength as temperature increases.
D.
E. The given spectrum could be explained using quantisation of energy
MEDIUM
JEE Main
IMPORTANT
The radius of the 2nd orbit of is . The expected radius of the 3rd orbit of is
MEDIUM
JEE Main
IMPORTANT
The number of given orbitals which have electron density along the axis is
MEDIUM
JEE Main
IMPORTANT
The shortest wavelength of hydrogen atom in Lyman series is . The longest wavelength in Balmer series of is
MEDIUM
JEE Main
IMPORTANT
Assume that the radius of the first Bohr orbit of hydrogen atom is . The radius of the third Bohr orbit of is _____ picometer. (Nearest Integer)
MEDIUM
JEE Main
IMPORTANT
The energy of one mole of photons of radiation of frequency in is _____ J .
(Nearest integer)
(Given: )
EASY
JEE Main
IMPORTANT
Maximum number of electrons that can be accommodated in shell with are:
