MEDIUM
Earn 100
If you are chilly outside the shower stall, why do you feel warm after bath if you stay in the bathroom?
Important Questions on Natural Resources
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




EASY
(Latent heat of ice is and )
HARD
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
MEDIUM
Statement (a) : When we pour little petrol on our palm, it causes the palm to feel cool.
Reason (b) : Particles of petrol evaporate using the energy from our palm which causes the palm to feel cool.
HARD

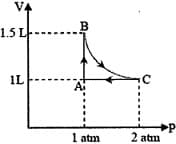
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
MEDIUM

MEDIUM
[Heat of fusion of ice ; Specific heat of water ]
EASY
Among the following gases, the order of liquefiability is
a)
b)
c)
d)
EASY
Which of the following gases can be absorbed in more proportion?
MEDIUM
EASY
EASY
HARD

The correct option(s) is (are)
EASY
EASY
EASY
MEDIUM
EASY
Let T = Temperature; H = Humidity and v = Wind speed
Which of the following are the best suited conditions for drying up of clothes?
EASY

