HARD
11th CBSE
IMPORTANT
Earn 100
Illustrate by taking examples of transition elements and non-transition elements that oxidation states of elements are largely based on electronic configuration.

Important Questions on Classification of Elements and Periodicity in Properties
HARD
11th CBSE
IMPORTANT
Nitrogen has positive electron gain enthalpy whereas oxygen has negative. However, oxygen has lower ionisation enthalpy than nitrogen. Explain.
HARD
11th CBSE
IMPORTANT
The first member of each group of representative elements (i.e., and -block elements) shows anomalous behaviour. Illustrate with two examples.
HARD
11th CBSE
IMPORTANT
-Block elements form acidic, basic and amphoteric oxides. Explain each property by giving two examples and also write the reactions of these oxides with water.
MEDIUM
11th CBSE
IMPORTANT
How would you explain the fact that first ionisation enthalpy of sodium is lower than that of magnesium but its second ionisation enthalpy is higher than that of magnesium?
EASY
11th CBSE
IMPORTANT
What do you understand by exothermic reaction and endothermic reaction? Give one example of each type.
MEDIUM
11th CBSE
IMPORTANT
Arrange the elements in the order of increasing first ionisation enthalpy. Give the reason for the arrangement assigned.
MEDIUM
11th CBSE
IMPORTANT
Arrange the elements in the order of increasing non-metallic character. Give the reason for the arrangement assigned.
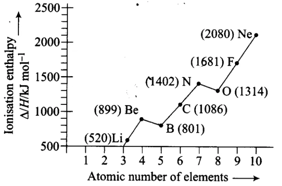
HARD
11th CBSE
IMPORTANT
Explain the deviation In Ionisation enthalpy of some elements from the general trend by using Figure