Iman and Arianna were given four different powders. They had to design a way of finding out if the powders were metals, carbonates or neither.
Here is their plan:
A: Add one spatula of powder to a test tube.
B: Add of hydrochloric acid to the test tube.
C: Fit a bung loosely on top of the test tube.
D: If the powder bubbles, remove the bung and hold a lighted splint close to the top of the tube.
E: Repeat steps A and B. Fit a bung with a delivery tube into the test tube — put the other end of the delivery tube into a test tube of limewater.
Here are their results:
Powder
Observations when the acid is added
Observations when a light splint was used
Observations when the gas was bubbled through limewater
1
Lots of bubbles produced quickly
Heard a pop noise
No change
2
No bubbles
No change
No change
3
A few bubbles produced slowly
Heard a pop noise
No change
4
Lots of bubbles produced quickly
Flame went out
Limewater went cloudy
The gas released in powder 4 turns the limewater milky. Which gas is it?
Here is their plan:
A: Add one spatula of powder to a test tube.
B: Add of hydrochloric acid to the test tube.
C: Fit a bung loosely on top of the test tube.
D: If the powder bubbles, remove the bung and hold a lighted splint close to the top of the tube.
E: Repeat steps A and B. Fit a bung with a delivery tube into the test tube — put the other end of the delivery tube into a test tube of limewater.

Important Questions on Sample Paper 2
The given reaction is:
In a double displacement reaction such as the reaction between sodium sulphate solution and barium chloride solution:
(A) exchange of atoms takes place.
(B) exchange of ions takes place.
(C) a precipitate is produced.
(D) an insoluble salt is produced.
The correct option is:
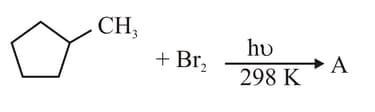
Identify the major product A:
On the basis of reactivity of different metals with oxygen, water and acids as well as displacement reactions, the metals have been arranged in the decreasing order of their reactivity. This arrangement is known as activity series of metals.
The basis of reactivity is the tendency of metals to lose electrons. If a metal can lose electrons easily to form positive ions, it will react readily with other substances. Therefore, it will be a reactive metal. On the other hand, if a meal loses electrons less rapidly to form a positive ion, it will react slowly with other substances. Therefore, such a metal will be less reactive.
Which of the following represents the correct order of reactivity for the given metals?
Incomplete combustion of coal and petroleum:
(A) increases air pollution.
(B) increases efficiency of machines.
(C) reduces global warming.
(D) produce poisonous gases.
The correct option is:
The below diagram shows the respiratory system of human beings.
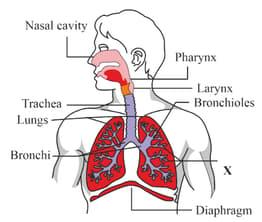
Identify the correct function of the part labelled as 'X'.

Organs and associated functions are given in options. Which of the following correctly states the function of the labelled organ?
