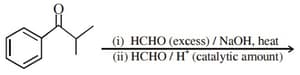EASY
Earn 100
Imine formation using an aldehyde/ketone and primary amine is acid-catalyzed, yet the rate drops below . Why does the rate drop below this ?
(a)The carbinolamine intermediate is stable at low pH
(b)The imine product is hydrolyzed at low pH
(c)Protonation of the amine decreases its nucleophilicity
(d)The amine is hydrolyzed at low pH
50% studentsanswered this correctly
Important Questions on Aldehydes and Ketones
MEDIUM
The major product of the following reaction is: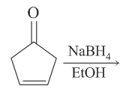
HARD
| Test | Inference |
| - test | Coloured precipitate yellow |
| Iodoform test | Yellow precipitate |
| Azo-dye test | No dye formation |
Compound is:
HARD
The major product of the following reaction is:
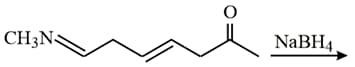
HARD
; the product B is:
MEDIUM
The major product 'X' formed in the following reaction is:
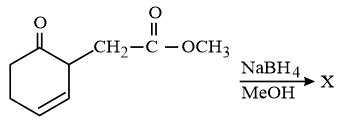
EASY
HARD
The major product obtained in the following reaction is:
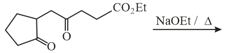
MEDIUM
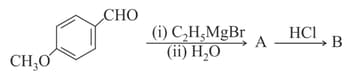
EASY
MEDIUM
In the following reactions, products and are:
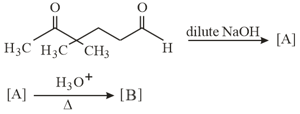
MEDIUM
In the following reactions
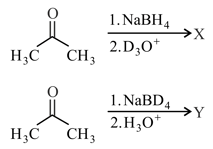
X and Y are
EASY
HARD
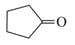 with methyl lithium gives which of the following species?
with methyl lithium gives which of the following species?MEDIUM
The increasing order of the reactivity of the following with is:
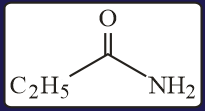
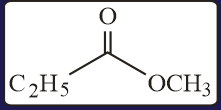
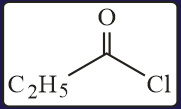
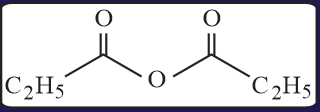
EASY
MEDIUM
EASY
In the reaction sequence
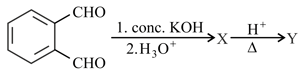
The major product X and Y, respectively, are
MEDIUM
The major product formed in the following reaction is:
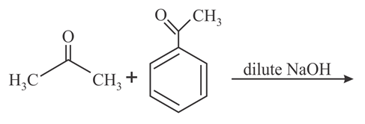
MEDIUM
HARD