HARD
Earn 100
In Carnot cycle, an ideal gas is taken through 4 reversible steps as shown in the diagram.
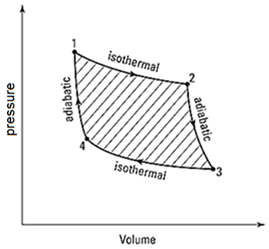
Which of the following statement(s) correct?
(i) ΔU is positive in the step between points 2 and 3.
(ii) ΔU is positive in the step between points 4 and 1.
(iii) ΔU is negative in the step between points 2 and 3.
(iv) Temperature at point 4 is higher than point 2.
(a)i, ii
(b)iv only
(c)ii, iii
(d)iii, iv
100% studentsanswered this correctly
Important Questions on Chemical Thermodynamics and Energetics
EASY
MEDIUM
Which of the following relations is correct?
HARD
MEDIUM
HARD
What will be the and values for the given cell having at and to be and respectively?
The cell: (Pt) (Given: Coulombs)
HARD
(Specific heat of water liquid and water vapour are and ; heat of liquid fusion and vaporization of water are and , respectively). ( )
EASY
An engine operating between the boiling and freezing points of water will have
A. Efficiency more than .
B. Efficiency less than the efficiency of a Carnot engine operating between the same two temperatures.
C. Efficiency equal to .
D. Efficiency less than .
Choose the correct answer from the options given below
EASY
MEDIUM
EASY
MEDIUM
Based on the above thermochemical equations, find out which one of the following algebraic relationships is correct?
MEDIUM
For the following reaction
and . What is the value of at ?
EASY
MEDIUM
HARD
HARD
Based on the above thermochemical equations, the value of at for the reaction
will be:
MEDIUM
EASY
EASY
HARD

