MEDIUM
Earn 100
In Mendeleev's periodic table some pairs of elements do not follow increasing order of atomic weight known as anomalous pair. The correct example of anomalous pair is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Periodicity
MEDIUM
EASY
Identify the incorrect match.
| Name IUPAC | Official Name |
| (a) Unnilunium | (i) Mendelevium |
| (b) Unniltrium | (ii) Lawrencium |
| (c) Unnilhexium | (iii) Seaborgium |
| (d) Unununium | (iv) Darmstadtium |
MEDIUM
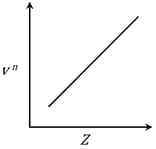
Given Frequency of X-ray emitted
Atomic number
EASY
MEDIUM
MEDIUM
MEDIUM
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power 'n' i.e. of X-rays emitted is plotted against atomic number , the following graph is obtained.
The value of 'n' is
EASY
EASY
MEDIUM
EASY
EASY
EASY
EASY
EASY
EASY
EASY
EASY
EASY
EASY