MEDIUM
Earn 100
In the
(a)Second bond in is formed by back bonding
(b) bond is formed by bonding
(c) bond is formed by bonding
(d) bond is formed by back bonding
50% studentsanswered this correctly
Important Questions on The p-Block Elements
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
EASY
The total number of and bonds present in the following compound are
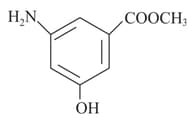
EASY
MEDIUM
MEDIUM
HARD

