MEDIUM
Earn 100
In Rutherford's experiment, a thin gold foil was bombarded by alpha particles. If Thomson's "Plum Pudding Model" of the atom were correct, what would have been the outcome of the experiment?
(a)Alpha particles would have got embedded in the foil.
(b)All the alpha particles would have been deflected from the foil.
(c)All the alpha particles would have been bounced back from the foil.
(d)Alpha particles would have passed through the foil with little or no deflection.
50% studentsanswered this correctly
Important Questions on Atoms
MEDIUM
EASY
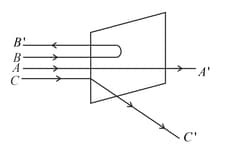
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
EASY
HARD
Consider the following statement:
(I) All isotopes of elements have the same number of neutrons.
(II) Only one isotope of an element can be stable and non-radioactive.
(III) All elements have isotopes.
(IV) All isotopes of Carbon can form chemical compounds with Oxygen.
The correct option regarding an isotope is:
EASY
Scattering angle
Number of scattered -particles detected
(Plots are schematic and not to scale)
EASY
HARD
EASY
EASY
MEDIUM
(Planck's constant electron mass )
MEDIUM
EASY
EASY
(Ignore radiation due to motion of electric charge)
EASY
EASY
MEDIUM
MEDIUM
HARD
EASY
Match List (Experiment performed) with List (Phenomena discovered/associated)and select the correct option from the options given below the lists
| List-I | List-II | ||
|---|---|---|---|
| Davisson and Germer experiment | Wave nature of electrons | ||
| Millikan's oil drop experiment | Charge of an electron | ||
| Rutherford experiment | Quantisation of energy levels | ||
| Franck-Hertz experiment | Existence of the nucleus |
EASY

