In a certain gaseous reaction between and The initial rates are reported as follows:
Rate
The rate law is.

Important Questions on Chemical Kinetics
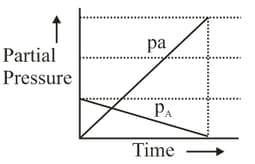
If for a reaction in which converts to the reaction carried out at constant results into the following graph.
In three different reactions, involving a single reactant in each case, a plot of the rate of the reaction on the -axis, versus the concentration of the reactant on the -axis, yields three different curves are shown below.
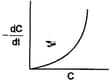
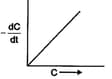
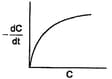
What are the possible orders of the reactions ?
A simple mechanism for the enzyme-catalysed reaction is given by the following set of equations:
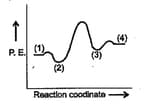
This is known as the Michaelis-Menten mechanism. The potential energy diagram is shown in the figure Which of the following sets of identifications is correct? (Assume that the temperature and pressure are constant).
