MEDIUM
MHT-CET
IMPORTANT
Earn 100
In a certain process, the pressure of an ideal gas varies with volume according to the relation , where and are constants. When the volume of one mole of gas is , the temperature of the gas will be,
(a)
(b)
(c)
(d)Zero
71.43% studentsanswered this correctly

Important Questions on Kinetic Theory
EASY
MHT-CET
IMPORTANT
In the figure, the volume of container is double of the volume of container . Both are filled with ideal gas. The temperature of is and that of is . If the mass of gas in is , then the mass of gas in will be?

MEDIUM
MHT-CET
IMPORTANT
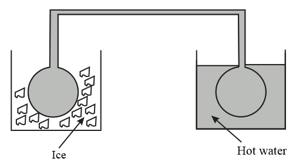
Two identical glass bulbs are interconnected by a thin glass tube at . A gas is filled in these bulbs. If one bulb is placed in ice and another bulb is placed in hot bath, the pressure of the gas become times. The temperature of hot bath will be.
MEDIUM
MHT-CET
IMPORTANT
On increasing the temperature of a gas filled in a closed container by , its pressure increases by , then initial temperature of the gas is:
MEDIUM
MHT-CET
IMPORTANT
A partition divides a container having insulating walls into two compartments. The same gas fills the two compartments. The parameters in the right compartment are and while in the left compartment, the corresponding parameters are and . Then the ratio of the number of molecules in the right compartment to that in the left compartment is
MEDIUM
MHT-CET
IMPORTANT
By what percentage should the pressure of a given mass of a gas be increased so as to decrease its volume by at a constant temperature?
MEDIUM
MHT-CET
IMPORTANT
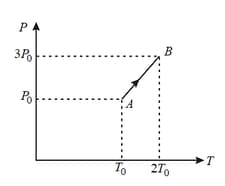
Pressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point is . Density at point will be:
EASY
MHT-CET
IMPORTANT
gas is filled up in a cylinder. If the pressure is increased two times, the temperature becomes four times, then the final density of the gas will be times the initial density.
EASY
MHT-CET
IMPORTANT
The constant quantity for equal volume of two gases at same pressure and temperature is,
