MEDIUM
JEE Main
IMPORTANT
Earn 100
In a cubic closed packed structure of mixed oxides, the lattice is made up of oxides ions, of tetrahedral voids are occupied by divalent ions and of the octahedral voids are occupied by trivalent ions. The formula of the oxide is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Solid State
MEDIUM
JEE Main
IMPORTANT
If radius of and radius of and is the unit cell edge length for crystal, then which of the given relation is correct?
MEDIUM
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
A compound has cubic close packing (CCP) arrangement of . Its unit cell structure is shown below. The empirical formula of the compound is
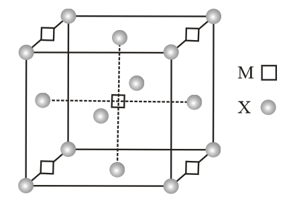
HARD
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
The unit cell with the following structure refers to______crystal system.
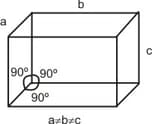
EASY
JEE Main
IMPORTANT
The crystal system of a compound with unit cell dimensions and is
