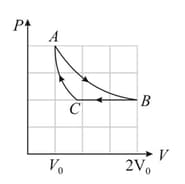
In a cycle consisting of isothermal expansion , isobaric compression and adiabatic compression , find the efficiency of cycle (Given: )


Important Questions on Thermodynamics
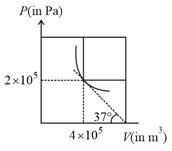
A container of volume is divided into two equal compartment by a partition. One of these compartments contains an ideal gas at . The other compartment is vacuum. The whole system is thermally isolated from its surroundings. The partition is removed and the gas expands to occupy the whole volume of the container. Find the new temperature.
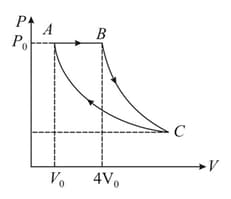
A fixed mass of a gas is taken through a process (as shown in the figure). Here is isobaric, is adiabatic and is isothermal. Find efficiency of the process (take ).
(a) Find the value of .
(b) What is the efficiency of the cycle?
Two Carnot's engines and are operated in series. The first one, , receives heat at and rejects to a reservoir at temperature . The second engine, , receives the heat rejected by the first engine and in turn rejects to a heat reservoir at . Calculate the temperature $T$ for the following situation:
The work outputs of the two engines are equal.
Two Carnot's engines and are operated in series. The first one, , receives heat at and rejects to a reservoir at temperature . The second engine, , receives the heat rejected by the first engine and in turn rejects to a heat reservoir at . Calculate the temperature $T$ for the following situation:
The efficiencies of two engines are equal.
A refrigerator freezes water at into ice at in a time interval of . Assuming the room temperature to be , calculate the minimum amount of power needed to make of ice
