In a hydrogen atom, an electron undergoes transition from the excited state to the excited state and then to the ground state. Identify the spectral series to which these transitions belong.

Important Questions on Bohr Model, X-Ray Spectra, Wave-Particle Duality
In a hydrogen atom, an electron undergoes transition from the second excited state to the first excited state and then to the ground state. Find out the ratio of the wavelengths of the emitted radiations in the two cases.
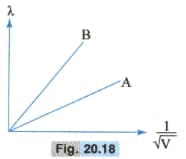
The two lines A and B, shown in the graph of Fig. , plot the de-Broglie wavelength as a function of for two particles, having the same charge . Which of the two represents particle of the heavier mass?
Draw a schematic diagram of the experimental arrangement used by Davisson and Germer to establish the wave nature of electrons. Explain briefly how the de-Broglie relation was experimentally verified in case of electrons.
