MEDIUM
12th West Bengal Board
IMPORTANT
Earn 100
In a hydrogen atom, an electron undergoes transition from the second excited state to the first excited state and then to the ground state. Find out the ratio of the wavelengths of the emitted radiations in the two cases.
Important Questions on Bohr Model, X-Ray Spectra, Wave-Particle Duality
EASY
12th West Bengal Board
IMPORTANT
What are ionisation energy, ionisation potential, excitation energy and excitation potential?
HARD
12th West Bengal Board
IMPORTANT
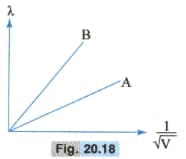
The two lines A and B, shown in the graph of Fig. , plot the de-Broglie wavelength as a function of for two particles, having the same charge . Which of the two represents particle of the heavier mass?
MEDIUM
12th West Bengal Board
IMPORTANT
Briefly explain dual nature of radiation?
HARD
12th West Bengal Board
IMPORTANT
Draw a schematic diagram of the experimental arrangement used by Davisson and Germer to establish the wave nature of electrons. Explain briefly how the de-Broglie relation was experimentally verified in case of electrons.
HARD
12th West Bengal Board
IMPORTANT
Explain why the idea of matter wave was proposed by deBroglie ? Why this idea was accepted?
HARD
12th West Bengal Board
IMPORTANT
Explain how the Bohr's postulate of quantisation of angular momentum may be derived from the idea of matter wave.
HARD
12th West Bengal Board
IMPORTANT
In a Geiger-Marsden experiment, what is the distance of the closest approach to the nucleus of an alpha particle before it comes momentarily to rest and reverse its direction? Given: Kinetic energy of -particle and for gold.
HARD
12th West Bengal Board
IMPORTANT
The total energy of an electron in the first excited state of the hydrogen atom is . What is the kinetic energy of the electron in this state?
