EASY
12th West Bengal Board
IMPORTANT
Earn 100
In a hydrogen atom, electron moves from second excited state to first excited state and then from the first excited state to the ground state. Find the ratio of the wavelengths obtained.

Important Questions on Bohr Model, X-Ray Spectra, Wave-Particle Duality
EASY
12th West Bengal Board
IMPORTANT
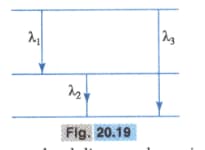
Find the relation between the three wavelengths and from the energy level diagram shown in the Fig. 20.19.
EASY
12th West Bengal Board
IMPORTANT
The characteristic line for X -rays from a certain element is found to be . Determine the atomic number of the element? Given .
HARD
12th West Bengal Board
IMPORTANT
Ultraviolet light of wavelength and , when allowed to fall on hydrogen atoms in their ground state, is found to liberate electrons with kinetic energies and respectively. Find the value of Planck's cosntant.
EASY
12th West Bengal Board
IMPORTANT
The ionisation energy of a hydrogen-like Bohr atom is rydbergs.
What is the wavelength of radiation emitted when the electron jumps from first excited state to ground state?
EASY
12th West Bengal Board
IMPORTANT
The ionisation energy of a hydrogen-like Bohr atom is Rydbergs.
What is the radius of the first orbit for this atom? Given that Bohr radius of hydrogen atom and Rydberg
EASY
12th West Bengal Board
IMPORTANT
A doubly ionized lithium atom is hydrogen-like with atomic number Find the wavelength of the radiation required to excite the electron in from the first to third Bohr orbit. The ionization energy of the hydrogen atom is
EASY
12th West Bengal Board
IMPORTANT
A doubly ionised lithium atom is hydrogen-like with atomic number . How many spectral lines are observed in the emission spectrum of the above exciting system?
EASY
12th West Bengal Board
IMPORTANT
The energy of an electron in an excited hydrogen atom is . Calculate the angular momentum of the electron according to Bohr's theory. Given : Rydberg's constant , Planck's constant , speed of light
