In a market with free entry and exit, what happens to economic profits in the long run?
Important Questions on Equilibrium
A solid kept in an evacuated sealed container undergoes decomposition to form a mixture of gases at temperature .
The equilibrium pressure is in this vessel. for this reaction is?
For the given reaction, if the initial pressure is and the pressure at time is at a constant temperature and constant volume . The fraction of decomposed under these conditions is . The value of is (nearest integer)
When and are compared at It is found that
.....(1)
.....(2)
The relation between and is:
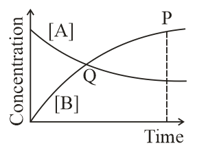
Among the following points indicated on the graph, which one represents equilibrium?
When mole of and mole of are mixed in a closed container at a constant temperature, mole of is obtained at equilibrium. Calculate the equilibrium amount of and .
In the reaction , which of the graphs is/are correct?
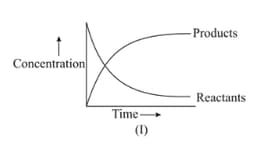
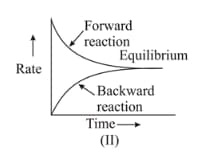
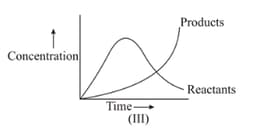
For the reaction , variation of concentration is plotted against time. The time at which the equilibrium establishes is as shown:
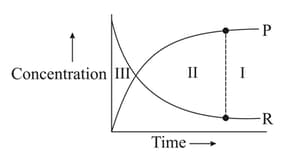
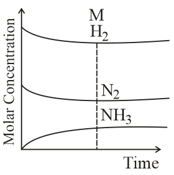
Which of the following regions show(s) equilibrium?
At equilibrium mixture for the reaction
2H2S ⇌2H2 + S2
had 1 mole of H2S, 0.2 mole of H2, and 0.8 mole of S2 in a
2 litre flask. The value of Kc in mol L- is