MEDIUM
Earn 100
In a reversible gaseous system, molar concentrations (active masses) of reactants or products are proportional to:
(a)partial pressure
(b)total pressure
(c)amounts of reactants and products
(d)none of the above
30% studentsanswered this correctly
Important Questions on Equilibrium
HARD
(At )
EASY
.....(1)
.....(2)
The relation between and is:
MEDIUM
MEDIUM
The equilibrium constant () of the reaction:
, will be:
EASY
HARD
MEDIUM
moles each of hydrogen and iodine is heated in a sealed ten litre vessel.At equilibrium, moles of were found. The equilibrium constant for is ........
EASY
(assuming ideality)
MEDIUM
The value of for the reaction
The value of for the following reaction is :
HARD
The reaction
is in equilibrium in a closed vessel at The partial pressure (in atm) of in the reaction vessel is closest to:
[Given: The change in Gibbs energies of formation at and bar for
Gas constant ]
MEDIUM
MEDIUM
When and are compared at It is found that
MEDIUM
.... (i)
.... (ii)
are related as
EASY
MEDIUM
HARD
When equal volumes of and solutions are mixed, the equilibrium concentration of is found to be The value of is __________ (Answer should be given to the nearest integer value).
MEDIUM
EASY
For the reaction:
is
EASY
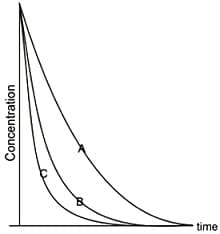
These profiles imply that the decay constants and follow the order
EASY
A solid kept in an evacuated sealed container undergoes decomposition to form a mixture of gases at temperature .
The equilibrium pressure is in this vessel. for this reaction is?

