EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
Earn 100
In a thermally isolated system. Two boxes filled with an ideal gas are connected by a valve. When the valve is in closed position, states of the box and . respectively, are and . When the valve is opened, the final pressure of the system is approximately.
(a)
(b)
(c)
(d)
53.85% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases and Thermodynamics
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
A coolant in a chemical or nuclear plant is a liquid that is used to prevent different parts of a plant from getting too hot. One important property of coolant is that it
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
A solid cube and a solid sphere of identical material and equal masses are heated to the same temperature and left to cool in the same surroundings. Then
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
In the Arctic region hemispherical houses called igloos are made of ice. It is possible to maintain inside an Igloo as high as because
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
An ideal gas filled in a cylinder occupies volume . The gas is compressed isothermally to the volume . Now the cylinder valve is opened and the gas is allowed to leak keeping temperature same. What percentage of the number of molecules escape to bring the pressure in the cylinder back to its original value.
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
An ideal gas follows aprocess described by from to ( is a constant). Then
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
One mole of a mono atomic ideal gas is expanded by a process described by where is a constant. The heat capacity of the gas during the process is given by ( is the gas constant)
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
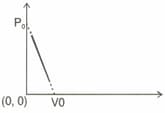
One mole of ideal gas undergoes a linear process as shown in the figure below. Its temperature expressed as a function of volume is-
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
A closed bottle containing water at is opened on the surface of the moon. Then
