EASY
JEE Advanced
IMPORTANT
Earn 100
In a very good vacuum system in the laboratory, the vacuum attained was . If the temperature of the system was , the number of molecules present in a volume of is
(a)
(b)
(c)
(d)zero
50% studentsanswered this correctly

Important Questions on Thermometry, Thermal Expansion and Kinetic Theory of Gases
EASY
JEE Advanced
IMPORTANT
If nitrogen gas molecule goes straight up with its speed at from the surface of the earth and there are no collisions with other molecules, then it will rise to an approximate height of
MEDIUM
JEE Advanced
IMPORTANT
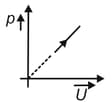
The given graph shows the variation of internal energy of an ideal gas with the increase in pressure. Which of the following pressurevolume graph is equivalent to this graph?
HARD
JEE Advanced
IMPORTANT
of gas is contained in a flask at a pressure of and at a temperature of . It is found that due to leakage in the flask, the pressure is reduced to half and the temperature to . The quantity of gas that leaked out is
MEDIUM
JEE Advanced
IMPORTANT
A mixture of of hydrogen and of helium at has a density about
EASY
JEE Advanced
IMPORTANT
The pressure and the density of the given mass of a gas expressed by Boyle's law, holds true
HARD
JEE Advanced
IMPORTANT
During an experiment, an ideal gas is found to obey a condition constant. (density of the gas). The gas is initially at temperature , pressure and density . The gas expands such that density changes to .
HARD
JEE Advanced
IMPORTANT
During an experiment, an ideal gas is found to obey a condition constant. The gas is initially at a temperature , pressure and volume . The gas expands to volume .
EASY
JEE Advanced
IMPORTANT
Find the correct options.