HARD
11th CBSE
IMPORTANT
Earn 100
In allene (), the type(s) of hybridization of the carbon atom(s) is/are.
(a) and
(b) and
(c)only
(d) and
50% studentsanswered this correctly

Important Questions on Organic Chemistry - Some Basic Principles and Techniques
HARD
11th CBSE
IMPORTANT
In the following compound the number of hybridized carbon is
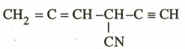 .
.
MEDIUM
11th CBSE
IMPORTANT
The state of hybridisation in of the hydrocarbon is
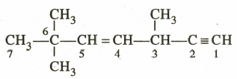 .
.HARD
11th CBSE
IMPORTANT
Which of the following molecules represent the order of hybridisation and from left to right atoms.
EASY
11th CBSE
IMPORTANT
In which of the following molecules, all atoms are coplanar?
HARD
11th CBSE
IMPORTANT
Considering the state of hybridisation of carbon atoms, find out among the following which is linear?
EASY
11th CBSE
IMPORTANT
The maximum number of carbon atoms arranged linearly in a molecule
HARD
11th CBSE
IMPORTANT
The number of and bonds present in ,-butadiene are respectively :
HARD
11th CBSE
IMPORTANT
In hexa--diene--yne, the number of , and bonds respectively are.
