In an Isothermal change, the change in pressure and volume of a gas can be represented for three different temperature; as:

Important Questions on Thermodynamics
Match List I with List II :
| List I | List II | ||
| A | Isothermal Process | I | Work done by the gas decreases internal energy |
| B | Adiabatic Process | II | No change in internal energy |
| C | Isochoric Process | III | The heat absorbed goes partly to increase internal energy and partly to do work |
| D | Isobaric Process | IV | No work is done on or by the gas |
Choose the correct answer from the options given below :
Given below are two statements. One is labelled as Assertion A and the other is labelled as Reason R.
Assertion A : If and represent the heat supplied to the system and the work done on the system respectively. Then according to the first law of thermodynamics .
Reason R : First law of thermodynamics is based on law of conservation of energy.
In the light of the above statements, choose the correct answer from the option given below :
Heat is given to an ideal gas in an isothermal process.
A. Internal energy of the gas will decrease.
B. Internal energy of the gas will increase.
C. Internal energy of the gas will not change.
D. The gas will do positive work.
E. The gas will do negative work.
Choose the correct answer from the options given below :
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A : Efficiency of a reversible heat engine will be highest at temperature of cold reservoir.
Reason R : The efficiency of Carnot’s engine depends not only on temperature of cold reservoir but it depends on the temperature of hot reservoir too and is given as
In the light of the above statements, choose the correct answer from the options given below :
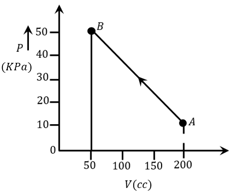
The pressure of a gas changes linearly with volume from to as shown in figure. If no heat is supplied to or extracted from the gas then change in the internal energy of the gas will be