EASY
Earn 100
In an electrochemical cell, the negative terminal of the battery is connected to-
(a)cathode
(b)anode
(c)both cathode and anode
(d)None of the above
50% studentsanswered this correctly
Important Questions on Electrolysis and Chemical Reaction
EASY
Consider the metals and solutions given in the box.
(a) Which of the above metals have to be selected to construct a Galvanic Cell?
(b)Identify the anode and cathode of the cell.
[Reactivity order ]
(c) Write the redox reaction taking place in the cell.
HARD
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
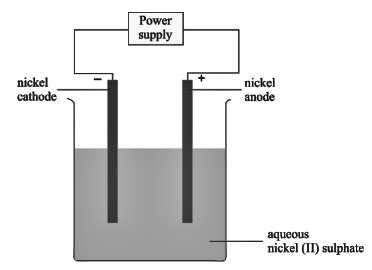
- Which equation for the reaction at the anode is correct?
EASY
EASY
EASY
(i) positively charged cathode
(ii) negatively charged anode
(iii) positively charged anode
(iv) negatively charged cathode
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
HARD
EASY
EASY
(a) Positively charged cathode
(b) Negatively charged anode
(c) Positively charged anode
(d) Negatively charged cathode
MEDIUM
EASY
(i) positively charged cathode
(ii) negatively charged anode
(iii) positively charged anode
(iv) negatively charged cathode
MEDIUM
MEDIUM
| Column I | Column II | ||
|---|---|---|---|
| (i) | Electrode connected to positive terminal of battery. | (a) | Cathode |
| (ii) | Electrode connected to negative terminal of battery. | (b) | Anode |
| (iii) | Substance which undergoes decomposition in aqueous solution, when current is passed | (c) | Non–electrolyte |
| (iv) | Substance which does not allow electric current to pass through it neither in aqueous not in molten state. | (d) | Electrolyte |
MEDIUM
HARD
HARD
| Cell | Instrument in which it is used |
| Dry cell | Radios |
| Cameras | |
| Clocks | |
| Toys | |
| Mercury cell | Watches |
| Calculator | |
| Electronic instruments | |
| Nickel- cadmium cell | Rechargeable torches |
| Cameras | |
| Lithium ion cell | Mobile phone |
| Laptops |
- What do we do with these types of cells after their use?
- Which among these cells can be recharged and reused?
- Do they cause environmental pollution?

