MEDIUM
JEE Advanced
IMPORTANT
Earn 100
In an ester molecule there are three bonds,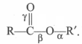 What do you expect regarding their relative bond length?
What do you expect regarding their relative bond length?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Carboxylic Acids and Their Derivatives
MEDIUM
JEE Advanced
IMPORTANT
The reason for greater reactivity of acetyl chloride for the nucleophilic substitution than methyl chloride is due to:
Capability of oxygen to acquire electrons.
Difference in the nature of carbon of the intermediate tetrahedral in case of acetyl chloride and a pentavalent in case of methyl chloride.
Difference in attack of nucleophile on the compound.
Better leavability of than .
MEDIUM
JEE Advanced
IMPORTANT
Which of the following compound undergoes Claisen condensation in presence of ?
(i)
(ii)
(iii)
HARD
JEE Advanced
IMPORTANT
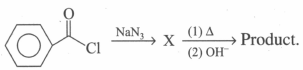
Product is/are:
HARD
JEE Advanced
IMPORTANT
Consider the following statements for hydrolysis reaction:
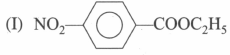 is more reactive than
is more reactive than
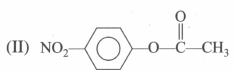 is more reactive than
is more reactive than 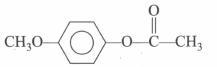
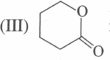 is more reactive than
is more reactive than 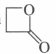
Of these the correct statements are
HARD
JEE Advanced
IMPORTANT
An organic compound gives a positive test with and phenolphthalein. Structure of X will be:
EASY
JEE Advanced
IMPORTANT
Which one of the following esters is the most reactive for saponification?
MEDIUM
JEE Advanced
IMPORTANT
Which of the following esters have the most acidic -hydrogen atoms?
HARD
JEE Advanced
IMPORTANT
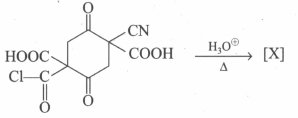
Select the incorrect statement:
