In an evacuated vessel of capacity moles of and moles of were introduced and the temperature is maintained at (assume that at this temperature decomposition of takes place). At equilibrium, the total pressure of the mixture was found to be . Correct statement(s) is/are

Important Questions on Equilibrium
The following represent the variation of with acid strength for aqueous solutions, Marked as and .
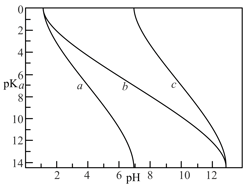
Select the correct matchings:
At , the progress of the reaction:
in a vessel is presented in the following figure:
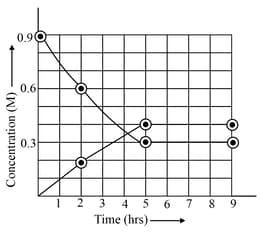
Identify the correct statement(s):
Determine the concentration of solution, one litre of which can dissolve of and of are and respectively.
How much of would remain in the solution after mixing equal volumes of and ?
If the answer is of type , report the value of correct up to nearest integer value.
Calculate (derived from ) to prevent from precipitating in a one-litre solution containing and ions.
[Answer has to be given after dividing with and correct up to one place of decimal.]
Calculate the solubility product of the reaction
Given that,
