MEDIUM
Earn 100
In carbonate ion, all three carbon - oxygen bonds are equivalent in all respects, viz, length, energy. What is the average bond order of carbon-oxygen?
(a)1.33
(b)0.123
(c)0.893
(d)1.92
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
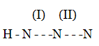
The above compound represents hydrogen azide, the bond orders of bonds and are:
MEDIUM
[Resonance energy of benzene
Enthalpy of hydrogenation of cyclohexene ]
EASY
MEDIUM
The molecule/molecules that has/have delocalised lone pair(s) of electrons is/are
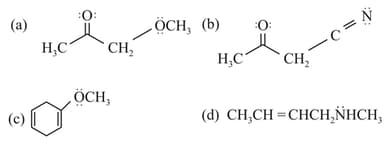
MEDIUM
Give a reason for the following:
Ionic compounds have a high melting point.
HARD
MEDIUM
EASY
EASY
MEDIUM
Resonance in carbonate ion is
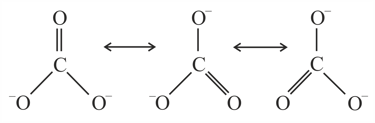
Which of the following is true?
MEDIUM
MEDIUM

