EASY
Earn 100
In context with beryllium, which one of the following statements is incorrect.
(a)It is rendered passive by nitric acid
(b)It forms
(c)Its salts rarely hydrolyze
(d)Its hydride is electron - deficient and polymeric
50% studentsanswered this correctly
Important Questions on The s-Block Elements
MEDIUM
MEDIUM
Given below are two statements:
Statement I : The chlorides of and have Cl-bridged structure. Both are soluble in organic solvents and act as Lewis bases.
Statement II: Hydroxides of and dissolve in excess alkali to give beryllate and aluminate ions. In the light of the above statements. Choose the correct answer from the options given below.
EASY
EASY
EASY
EASY
EASY
HARD
MEDIUM
EASY
Among the statement (I – IV), the correct ones are:
(I) Be has smaller atomic radius compared to Mg.
(II) Be has higher ionization enthalpy than AI.
(III) Charge/radius ratio of Be is greater than that of Al.
(IV) Both Be and Al form mainly covalent compounds
MEDIUM
HARD
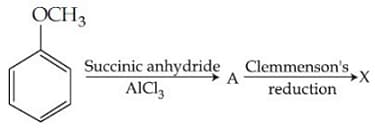
X is :
MEDIUM
MEDIUM
MEDIUM
MEDIUM
Reaction of with gives :
(A)
(B)
(C)
(D)
(E)
Choose the correct answer from options given below
MEDIUM
EASY
Reason (R) : Both and have almost same ionic radius
The correct option among the following is
EASY
EASY

