EASY
10th CBSE
IMPORTANT
Earn 100
In electron dot structure, the valence shell electrons are represented by crosses or dots.
The atomic number of chlorine is . Write its electronic configuration.

Important Questions on Carbon and its Compounds
EASY
10th CBSE
IMPORTANT
In electron dot structure, the valence shell electrons are represented by crosses or dots.
Draw the electron dot structure of chlorine molecule.
MEDIUM
10th CBSE
IMPORTANT
Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
MEDIUM
10th CBSE
IMPORTANT
Unsaturated hydrocarbons contain multiple bonds between the two C-atoms and show addition reaction. Give the test to distinguish ethane from ethene.
MEDIUM
10th CBSE
IMPORTANT
Match the reactions given in Column I with the names given in Column II.
| Column I | Column II | ||
| A. | 1. | Addition reaction | |
| B. | 2. | Substitution reaction | |
| C. | 3. | Neutralisation reaction | |
| D. | 4. | Esterification reaction |
HARD
10th CBSE
IMPORTANT
Write the structural formulae of all the isomers of hexane.
EASY
10th CBSE
IMPORTANT
What is the role of metal or reagents written on arrows in the given chemical reactions?
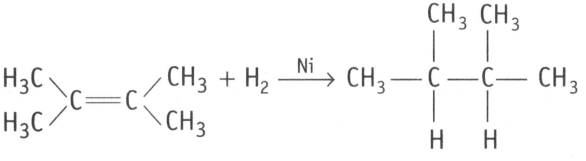
EASY
10th CBSE
IMPORTANT
What is the role of metal or reagents written on arrows in the given chemical reaction?
EASY
10th CBSE
IMPORTANT
What is the role of metal or reagents written on arrows in the given chemical reaction?
